Background
In the past 50 years, there has been an increase in Implantable Medical Devices (IMDs). IMDs are often powered by batteries, with lithium-based batteries being the most common selection due to their relatively long lifetime of 5 to 10 years. Since patients often outlive the lifespan of the battery, surgical replacement of either the battery or the entire device is often required. This introduces additional costs and risks to the patient. The objective of this project is to develop a fully implantable electric generating/charging system to prevent the need for surgical battery replacements in various IMDs.
Requirements
Customer Requirements (CRs) Given the vague project description, the team was required to interpret and determine its own customer requirements. W.L. Gore suggested many overall requirements that were analyzed and are acknowledged within the list of customer requirements. These requirements are as follows:First off, the team decided that meeting regulatory safety standards is the most important requirement to be fulfilled. This requirement is weighted 45 out of 100. Secondly, weighted 35 out of 100, the final product will have to provide electric power. Finally, the lowest weighted requirement, but still important, is the device's ability to be fully implanted within the human body. This requirement fulfills the last 20 weighting points and is the final customer requirement for the project. To better illustrate specific needs, these three customer requirements were broken down further.
- Meet regulatory standards
- Provide generated electric power
- Achieve full implantability within the human body
Target Specifications (ERs) The engineering requirements have been developed to satisfy the specifications of a Medtronic Advisa DR MRI SureScan A2DR01 pacemaker. The team established ranges for a majority of the engineering requirements to increase the chance of success. Power requirements include target specifications for open voltage, control minimum voltage, control max current, life span, and pacing rate. Size requirements include target specifications for weight and volume. The team determined values for power and size using the Medtronic specification sheet. Material requirements include target specifications for biocompatibility and cycle life. The team decided the target material must exceed a 10-year cycle life because the A2DR01 pacemaker's projected service life is 10 years. The team established a target specification for a location customer requirement as well. Since each bodily organ and joint consumes a set amount of power, the team decided the selected organ or joint must consume greater than 10 mW of power. The value assumes the team can pull greater than 0.1% of the organ's power. Implant method will correlate to an industry accepted method and biocompatibility will be based on the selected material's bending stiffness. Justified by a comparable Dacron Patch, the bending stiffness must be less than 0.00252 newton-meters. Listed below are all the current engineering requirements and their associated values.
| Requirement | Target | Tolerance |
|---|---|---|
| Size | 500 cm^3 | Less than |
| Location | 1.5 m from the IMD | Less than |
| Charging Rate | 10 μW | Greater than |
| Cost | $3000 | ± $2000 |
| Reliability | 80% | +20%, -10% |
| Durability | 2 hours with < 30% decrease in power | Less than |
| Weight | 280 grams | Less than |
Models
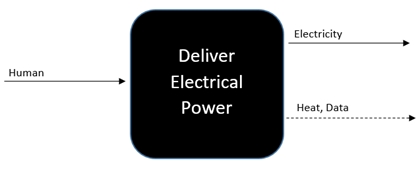
Based upon the project requirements, the team decided the overall goal of the project was to “deliver electrical power.” Therefore, this goal was placed in the center of the black box model since there is no material or signal that will be input into the device, human energy is the only input displayed. Electricity is the only energy that will be output and heat and data are the two outputted signals. This black box model helped the team to clarify the project. The simplified decomposition of the device provides a platform for further thought to be placed upon each input and output. This is shown in Figure 1.
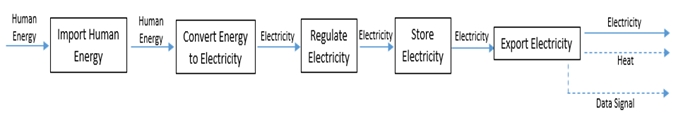
In this case, the defined scope is energy and signal flow. The solid lines in the functional model below all represent some form of energy flow, whereas the dashed lines represent signal flow. This model is designed to help visualize the function and process of the design as well as to help identify opportunities and possible costs. The visual representation allows for a deeper understanding of the system which allows for expansion on prior ideas. This is shown in Figure 2.
Power Generation
Piezoelectricity is electricity that results from pressure and heat. When mechanical stress is applied to certain solid materials, an electric charge accumulates within it and can be harnessed as an energy source. The piezoelectric effect is a reversible process. Materials like quartz and Rochelle salt are two natural occurring crystals that exhibit a piezoelectric effect upon application of stress. Biological materials such as a tendons and enamel exhibit the same phenomenon. Especially interesting is a polymer caller fluoride because the polymer material exhibits piezoelectricity several times greater than quartz.
Energy Storage
The storage of the electricity that is generated by the piezoelectric generator will be the responsibility of the specific super capacitor the team chooses to employ. The team chose to avoid traditional battery and rechargeable battery options because of the average 10-year life span they exhibit. However, this means that wherever the team decides to place the piezoelectric generator, the site in the human body must deform often to keep the supercapacitor charged. For this reason, places like the toes or fingers have been disregarded because deformation would depend on the patient's activity levels. The heart, diaphragm, or shoulder joint deforms more frequently and for the heart and diaphragm, the deformation is rhythmic. Rhythmic or frequent deformation would assure that the supercapacitor is always charged and the pacemaker always able to function.