Project Background
Catheter balloons are used for a range of medical procedures that require narrow passages or openings within the body to be enlarged. Once inserted into the body, these specialty balloons inflate to enlarge narrow openings or passages that are too small for doctors to work in. Many different procedures utilize catheters balloons ranging from the replacement of heart valves to treating torn ducts in the eye. POBA Medical currently manufactures a variety of catheter balloons and desire a device that will efficiently measure and detect any defects. In order for these catheter balloons to be used during procedures, each balloon inspected must have adequate dimension and no defects. With research and design testing we have created an automated visual balloon inspection device that will examine the entire catheter balloon that will reveal the dimensions and any defects that may be present. With this device, POBA Medical will be able to measure all their different types of catheter balloons, and optimize their manufacturing process.
Project Location
Our team project took place on the Northern Arizona University Campus in Flagstaff, Arizona (FIgure 1). This was the primary location for most meetings and where our device was assembled.
Figure 1-Northern Arizona University location in Flagstaff, Arizona.
Project Constraints
Project Budget: $11,000
Project Length: 9 months
Engineering Requirements
Our team was tasked with creating a device that can:
-Measure all outer balloon dimensions. (Proximal and distal neck OD, cone length and angle, working length diameter and length).
-Measure size of visual defects (“Gel Spots”, ”Fish eyes”, ”Crows Feet”, etc. ).
-Measure distance between visual defects and number of defects within a specified area.
-Rotation for inspection.
-Longitudinal movement for inspection.
-Inexpensive.
-Inflation for inspection (Equipment must be able to withstand 40 ATM of internal pressure).
-Equipment will be able to sit on a table.
Project Schedule
Design Considerations
Figure 2 shows an early solidworks model of our device. There are some missing components to this design because subsystems were not introduced yet or they were not complete. The sections below has two empty ports that were intended to hold the motion contrller for the horizontal and vertical stages.
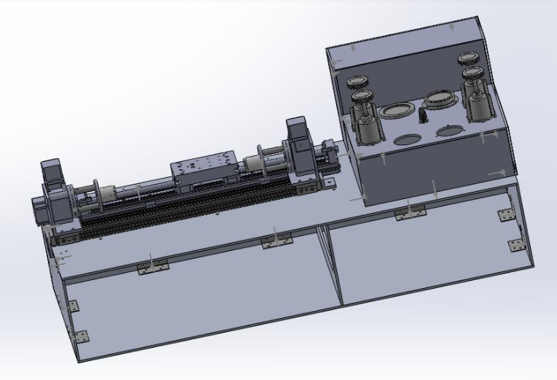
Figure 2-Early design of device.
Figure 3 is our final design. This design includes all subsystems necessary for our device to meet our engineering requirements. Despite a late budget cut to the theam budget ($20,000 cut to $11,000), our device was altered so that components that were too expensive could be purchased and attached in the future.
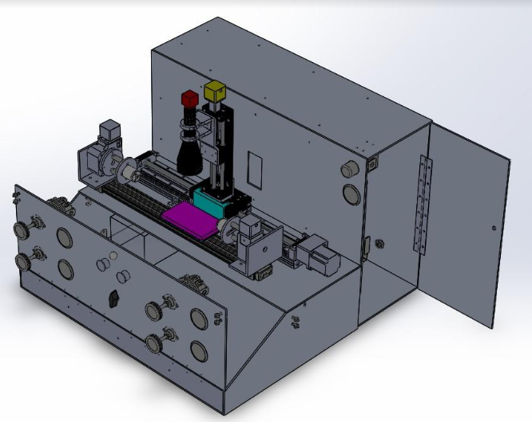
Figure 3-Refined/Final design of device.
Decision Matrix
Figure 4 shows the design matrix used when selecting our device. Thefinal design selected had the highest score for the decision matrix as well as being the favored design by the entire team. This design was favored in that it was easy to incorporate all moving sections of the design. This design was also favored by our client, POBA medical.
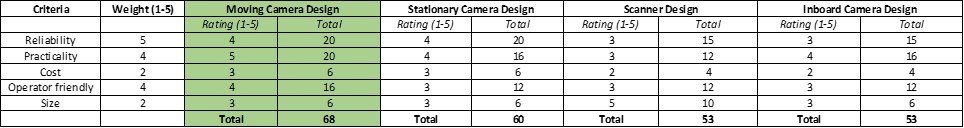
Figure 4-Decision Matrix
Details of Final Design
Figure 5 shows the final fabricated design for our device.

Figure 5- Final Design